Alzheimer’s Disease Research: Breakthroughs by Beth Stevens
- admin
- 0
- Posted on
Alzheimer’s disease research is at the forefront of neurological discoveries, offering insights that can transform lives. Each year, millions grapple with this debilitating condition, prompting scientists to explore the role of microglial cells in the brain’s immune response. Beth Stevens, a pioneering neuroscientist, has made significant strides in understanding how these cells contribute to synapse pruning, a vital process that, when mismanaged, plays a critical role in the progression of Alzheimer’s. With her pioneering work, Stevens is uncovering new Alzheimer’s biomarkers that could lead to earlier diagnoses and more effective neurodegenerative disease treatments. As we stand on the brink of breakthroughs in Alzheimer’s research, the potential to change the landscape of care for millions is more significant than ever.
Investigating the complexities of dementia is essential as researchers continue to unveil different aspects of Alzheimer’s pathology. The scientific community is now diving deeper into the intricate functions of glial cells, particularly how these immune-like cells in the brain help manage synaptic health. With the insights from renowned figures like Beth Stevens, the dialogue around Alzheimer’s extends beyond mere symptom management to the development of innovative therapeutic strategies. Cutting-edge studies aim to identify reliable biomarkers that could significantly alter the early detection process, enhancing patient care options. As we broaden our understanding of Alzheimer’s and its underlying mechanisms, pressing forward can lead to revolutionary treatments that resonate throughout the healthcare landscape.
Understanding Microglial Cells in Alzheimer’s Disease
Microglial cells serve as a crucial part of the brain’s immune system, constantly surveying the central nervous system for signs of injury or disease. In the context of Alzheimer’s disease, these cells play a dual role: they are responsible for maintaining neuronal health by clearing up cell debris and facilitating synaptic pruning, which is essential for proper neural function. However, Beth Stevens’ research has revealed that when microglial cells malfunction, it can lead to excessive pruning of synapses. This abnormal pruning is believed to contribute significantly to the pathogenesis of Alzheimer’s and other neurodegenerative diseases.
The discoveries made by Stevens and her team have profound implications for the future of Alzheimer’s disease research and treatment. By understanding the mechanisms behind microglial activity, scientists can pinpoint specific dysfunctions that may serve as biomarkers for early detection of Alzheimer’s. Furthermore, targeting the pathways involved in microglial activation and synaptic pruning could lead to new therapeutic strategies aimed at preventing or slowing down the progression of this debilitating disease.
Beth Stevens: Pioneering Neuroimmune Research
Beth Stevens has emerged as a leading figure in neuroimmune research, particularly in understanding how microglial cells influence the development of neurodegenerative diseases. Her work has evolved significantly since her early career, driven by a deep curiosity about the brain’s immune response during normal development and disease processes. With significant federal funding backing her research, Stevens has been able to explore complex questions regarding microglial functions and their relationship with neuronal circuits. Such foundational research is essential, as it lays the groundwork for translating science into practical applications for the treatment of conditions like Alzheimer’s.
Stevens’ contributions have also highlighted the interconnections between curiosity-driven research and clinical applications. Although early findings might seem tangential to disease treatment, they often unveil pathways that can be exploited for therapeutic gains. By examining how microglial cells operate during both healthy and pathological states, Stevens not only advances our understanding of Alzheimer’s but also paves the way for potential interventions aimed at enhancing brain health in aging populations.
Advancements in Alzheimer’s Disease Biomarkers
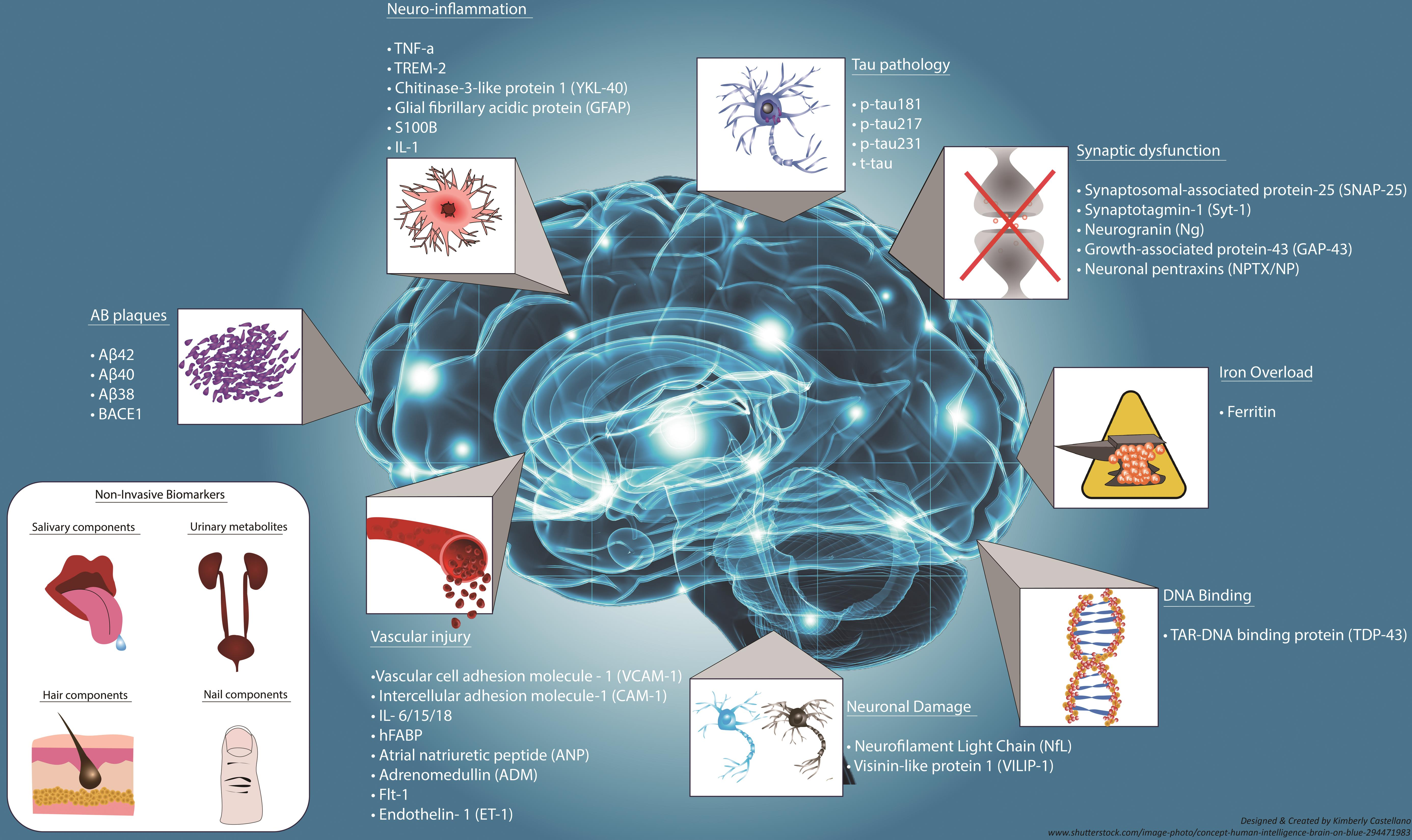
Alzheimer’s disease biomarkers are critical for early diagnosis and tracking disease progression, and Stevens’ research contributes significantly to this field. By examining the activities of microglial cells and their role in synaptic pruning, Stevens has identified potential biomarkers that can indicate the onset of Alzheimer’s before significant symptoms appear. These biomarkers can facilitate earlier interventions, providing patients access to treatments that could slow the disease’s progression and improve their quality of life.
As the understanding of Alzheimer’s biomarkers evolves, it empowers healthcare professionals to be proactive in patient care. Identification of these biomarkers can lead to personalized treatment plans that encompass various therapeutic approaches, including lifestyle changes, medication, and support mechanisms. The ability to detect neurodegenerative changes earlier can transform the management of Alzheimer’s disease, potentially reducing the burden on patients and healthcare systems alike.
The Impact of Neurodegenerative Disease Treatments
The development of effective neurodegenerative disease treatments is a pressing global health challenge, particularly as the population ages. Stevens’ research provides key insights into potential interventions targeting microglial function, thus addressing the underlying mechanisms of diseases like Alzheimer’s. Therapeutics that can modulate microglial activity may help restore normal synaptic pruning, potentially halting or reversing cognitive decline caused by Alzheimer’s.
While there are currently limited treatments that significantly alter the course of Alzheimer’s, ongoing research inspired by Stevens’ findings is paving the way for innovative approaches. The focus on neuroimmune interactions offers a promising avenue for drug development that could lead to breakthroughs in how we treat and manage Alzheimer’s disease, improving outcomes for millions of individuals affected by this devastating condition.
Exploring Neuroimmune Interactions
Neuroimmune interactions are at the forefront of research into neurodegenerative diseases, with microglial cells forming a critical link between immune response and neuronal health. Stevens’ work emphasizes the importance of understanding these interactions to develop targeted treatments for Alzheimer’s and similar conditions. By exploring how microglia communicate with neurons and influence synaptic dynamics, researchers can uncover new strategies for therapeutic intervention.
This area of study not only holds potential for Alzheimer’s disease but also extends to other neurodegenerative disorders like Huntington’s and multiple sclerosis. Insights gained from Stevens’ research can lead to the discovery of shared pathways and therapeutic targets across these conditions, underscoring the interconnectedness of neurological health and immune function.
Funding the Future of Alzheimer’s Research
Funding is a crucial factor in advancing Alzheimer’s disease research, enabling scientists like Beth Stevens to pursue innovative lines of inquiry. The support from federal agencies, particularly the National Institutes of Health, has been instrumental in facilitating groundbreaking work on microglial cells and their implications for Alzheimer’s. Such financial backing not only accelerates research progress but also helps attract new talent to the field, fostering an environment of discovery.
Increased investment in Alzheimer’s research is vital, especially as projections indicate a doubling of cases in the coming decades. Sustaining funding will ensure that researchers can continue to explore pressing questions related to microglial dysfunction and its role in Alzheimer’s progression. As knowledge expands, so too will the possibilities for developing effective treatments that can ultimately lessen the burden of this disease on individuals and healthcare systems.
Translational Science and Alzheimer’s Treatment
Translational science plays a pivotal role in bridging basic research and clinical application, and Beth Stevens’ work exemplifies this approach in Alzheimer’s disease. By translating discoveries about microglial cells into potential therapies, researchers can create more effective treatment strategies that address the root causes of neurodegeneration. This seamless transition from lab to clinic is essential for developing interventions that can genuinely improve patient outcomes.
Stevens’ efforts demonstrate that insights gained from foundational studies can lead to profound advancements in treating Alzheimer’s. As researchers continue to translate complex neurobiological findings into practical applications, the landscape of Alzheimer’s treatment may shift dramatically, offering hope to millions who are affected by this debilitating disease.
Navigating the Challenges of Alzheimer’s Research
Conducting research on Alzheimer’s disease presents a myriad of challenges, from the complexity of its pathology to the intricacies of studying human cognition. Stevens acknowledges that navigating these obstacles requires a blend of persistence and innovative thinking. Her lab’s focus on the basic science of microglial function has created a solid foundation necessary for tackling these challenges, providing a roadmap for understanding how alterations in neuroimmune systems contribute to Alzheimer’s.
Despite the hurdles, the dedication to uncovering the mysteries of Alzheimer’s continues to be fueled by the possibility of transformative discoveries. As researchers like Stevens illuminate the interactions between microglial cells and neuronal health, they are positioned to make meaningful strides in treating and preventing Alzheimer’s disease, ultimately changing the narrative for those at risk or living with its repercussions.
The Future of Alzheimer’s Disease Research
The future of Alzheimer’s disease research is bright, fueled by an increasing focus on understanding the mechanisms underlying neurodegeneration and the role of microglial cells. As studies continue to reveal the complexities of the brain’s immune system, researchers will be better equipped to develop targeted therapies that address Alzheimer’s at its core. The emphasis on curiosity-driven exploration will remain vital, as unexpected discoveries often lead to the most innovative solutions.
Looking ahead, the integration of advanced technologies such as genomics, imaging, and artificial intelligence into Alzheimer’s research will enhance our ability to identify biomarkers and validate potential treatments. As the landscape of neurological discoveries expands, collaboration among scientists, clinicians, and funding bodies will be essential in delivering effective strategies to combat Alzheimer’s disease and improve the lives of those affected.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are crucial in Alzheimer’s disease research as they act as the brain’s immune system, monitoring neuronal health, and removing dead cells. Beth Stevens’ research highlights how improper functioning of these cells, particularly in the pruning of synapses, may contribute to the progression of Alzheimer’s and other neurodegenerative diseases.
How are Alzheimer’s biomarkers identified in current research?
Current Alzheimer’s disease research focuses on identifying biomarkers that can detect the disease in its early stages. These biomarkers, influenced by findings from studies like those conducted by Beth Stevens, help scientists understand the mechanisms of Alzheimer’s, such as microglial dysfunction and neuroinflammation, allowing for earlier diagnosis and intervention.
What are some neurological discoveries impacting Alzheimer’s disease?
Neurological discoveries impacting Alzheimer’s disease include advancements in understanding the role of microglial cells and their effects on synaptic pruning, as investigated by researchers like Beth Stevens. These findings are paving the way for new treatment options and improved methods to diagnose Alzheimer’s through various biomarkers.
What advancements have been made in neurodegenerative disease treatments through Alzheimer’s research?
Advancements in neurodegenerative disease treatments have emerged from Alzheimer’s research that focuses on microglial cells, their roles in immune response, and synaptic health. Scholars like Beth Stevens have laid the groundwork for innovative therapies targeting the underlying mechanisms of Alzheimer’s, potentially leading to more effective care for affected individuals.
How does Beth Stevens’s research contribute to understanding Alzheimer’s disease?
Beth Stevens’s research significantly enhances our understanding of Alzheimer’s disease by revealing the functions of microglial cells in the brain. Her studies illustrate how these cells can both protect and negatively impact neuronal health, highlighting pathways that could be targeted for developing treatments and early detection methods for Alzheimer’s.
| Key Point | Details |
|---|---|
| Microglial Cells | Act as the brain’s immune system, removing dead cells and pruning synapses. |
| Impact on Alzheimer’s | Improper pruning of microglia can contribute to Alzheimer’s and other diseases. |
| Research Support | Funded mainly by NIH and federal agencies, enabling significant research. |
| Future Implications | Research has potential for new treatments and early detection biomarkers for Alzheimer’s. |
| Aging Population | Projected doubling of new Alzheimer’s cases by 2050, increasing care costs. |
Summary
Alzheimer’s disease research continues to make significant strides thanks to innovative scientists like Beth Stevens, who emphasize the importance of microglial cells in brain health. Their groundbreaking work not only enhances our understanding of the mechanisms behind neurodegenerative diseases but also paves the way for developing effective treatments and early detection methods for millions affected by Alzheimer’s. As the population ages, the urgency for continued Alzheimer’s disease research becomes increasingly vital to address the looming healthcare crisis.